
New MiDOG Research on Feline Stomatitis
Does your cat’s “meowth” look red and swollen? If so, your friend may be suffering from feline chronic gingivostomatitis (FCGS), which is a painful and chronic inflammatory disease of the oral cavity that affects 0.7% to 12% of cats in their lifetime [1]. FCGS has a multifactorial etiology caused by a “hyper” immune response to one or more initial triggers.
Feline chronic gingivostomatitis is uncomfortable at best for your furry friend, and if left untreated may result in serious health complications. Current treatment for FCGS is variable, with treatment often requiring partial or full mouth tooth extractions [2]. Fortunately, new research using Next-Gen Sequencing (NGS) technology has provided exciting insights into the oral microbiome of cats and the occurrence of antimicrobial resistance genes in the oral cavity of cats with FCGS, which could have substantial impacts on how veterinarians treat the disease [3]. If you suspect your cat has FCGS, it is recommended that you make an appointment with your veterinarian to diagnose and provide a treatment plan tailored to your cat’s needs as soon as possible.
What is Feline Gingivostomatitis?
Feline chronic gingivostomatitis refers to the characteristic longitudinal presentation of oral mucosal ulcerative and/or proliferative inflammatory lesions lateral to the cat’s palatoglossal folds [2]. While the etiology of FCGS is still the subject of intensive investigations in veterinary medicine, various infectious agents have been associated with FCGS pathogenesis: feline herpesvirus (FHV-1), feline calicivirus (FCV), feline immunodeficiency virus (FIV), feline leukemia virus (FeLV), various bacteria, and various noninfectious factors (dental disease, environmental stressors, hypersensitivity, etc.) [2]. In terms of predisposing factors, one study revealed that there seems to be an association between multi-cat households and FCGS pathogenesis, with each additional cat increasing the odds of a cat developing FCGS by over 70% [4].
The feline microbiome potentially plays an important role in FCGS manifestation, with the feline oral microbiome having less diversity in FCGS-affected cats versus healthy cats [2]. Moreover, the species Pasteurella multocida, phylum Bacteroidetes, and genus Peptostreptococcus are more common in FCGS cats than healthy ones [2,5]. Bartonella infections (cat scratch fever) are also considered by veterinarians when trying to diagnose your cat [6]. In general, Gram-negative and anaerobic bacteria are implicated with feline chronic gingivostomatitis, although current research is attempting to elucidate if this relationship is causative or simply associated with FCGS’s pathogenesis [2]. Regardless, treating the infection, whether it be causative or associated with FCGS, is necessary.
For any cat parent, identifying symptoms of feline gingivostomatitis is critical. Symptoms may include:
- Extreme oral pain
- Swollen, ulcerated, and bleeding gums
- Bar breath
- Blood in saliva
- Drooling
- Lack of appetite and/or weight loss
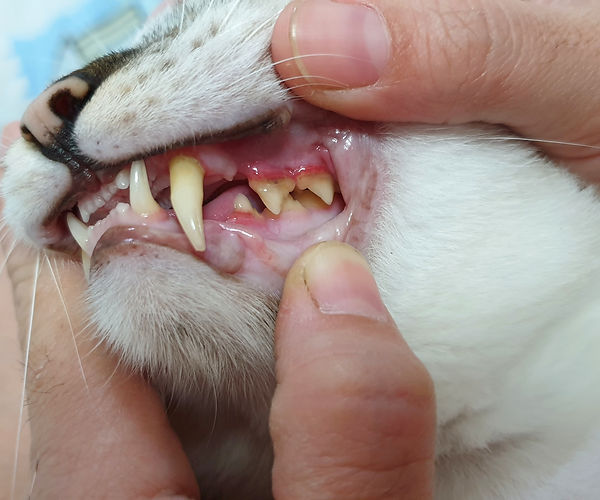
The image above depicts the swollen gums associated with FCGS.
Treating and Diagnosing Feline Chronic Gingivostomatitis
The prognosis for cats suffering from FCGS is guarded, considering that approximately 30% of cats do not respond to full mouth tooth extractions [1]. While research suggests partial tooth extractions may be equally as effective as full tooth extractions, it is important to consider possible microbial causes of FCGS when treating your cat [7]. Antibiotic therapy is often included in an FCGS-afflicted cat’s treatment plan, although this without a proper understanding of the potential infection at hand may be counterintuitive. With the rise of antibiotic-resistant strains of pathogens, advances in recent clinical diagnostics have allowed for more targeted and efficient interventions. Empirical treatment of feline chronic gingivostomatitis with amoxicillin, amoxicillin clavulanate, clindamycin, or metronidazole could contribute to this human and veterinary health dilemma [3].
Broad-spectrum antibiotics can impact not only your cat’s microbiome health but also the surrounding environment. Often times broad spectrum antibiotics are prescribed empirically in cases with FCGS, without culture and sensitivity analysis [8]. This not only increases risk if reinfection, but is a public health issue for both animals and humans. Several studies have shown that unused/unmetabolized antibiotics are disposed in the trash or excreted as waste, with many of these unused antibiotics leeching into surrounding waterways and the environment. Wasted antibiotics have resulted in a drastic rise in multi-drug resistant bacteria in our food, environment, and hospitals. Therefore, understanding the microbial makeup of your cat’s infection is of the utmost importance.
Recent Advancements in Feline Chronic Gingivostomatitis Diagnostics
Historically, culture-based methods have been used to assess the oral microbiome of cats, but there are notable diagnostic shortcomings. One recent study characterizing the oral microbiome of healthy cats using NGS technology notes “the tendency of culture [based methods] to overestimate the importance of species that are easily cultivated, and underestimate the fastidious organisms that grow poorly on culture media” [9]. This study goes on to support the potential usage of molecular diagnostics for feline oral disorders, as results revealed a far more diverse oral microbiome in cats than was previously established [9].
Next-Gen Sequencing (NGS) has increasingly helped researchers and veterinarians characterize the feline oral microbiome. In a past collaboration between Western University School of Veterinary Science and MiDOG, one of the first studies was conducted showing that fungi may play a larger role in the initiation and progression of feline chronic gingivostomatitis, as Malassezia restricta, Malassezia arunalokei, Cladosporium penidielloides/salinae, and Aspergillaceae sp. were significantly enriched in cats with FCGS [3]. Conversely, Saccharomyces cerevisiae was enriched in clinically healthy cats [3]. This research not only supports the use of NGS when attempting to diagnose potential FCGS cases but also indicates a more complex interaction between feline oral bacterial and fungal populations than was previously thought.
Read the study here to learn more.
Recently, this collaboration has produced exciting new research exploring the differential occurrence of antimicrobial resistant (AMR) bacterial genes and the co-occurrence of AMR genes with oral fungal species. With the help of MiDOG NGS technology, a total of 21 and 23 different AMR genes were detected in CH and FCGS cats, respectively [8]. Moreover, two AMR genes (mecA and mphD) showed statistically significant co-occurrence with Malassezia restricta [8]. These results are extremely important to consider in a clinical context when determining appropriate treatment regimens and risk for re-infection.
Read the study here to learn more.
The MiDOG All-in-One Microbial Test offers comprehensive diagnostic information for cats suffering from feline chronic gingivostomatitis. Utilizing NGS technology to detect and quantify all microbial DNA through untargeted and comprehensive sequencing and quantitative comparisons to reference databases, the MiDOG NGS technology provides a useful opportunity to shed light on the microbial makeup of your cat’s infection for clinical application. The MiDOG microbiome test is a microbial identification test grounded on scientific research that provides veterinarians DNA evidence for the guided treatment of cat diseases, such as feline chronic gingivostomatitis.

Find out if your vet uses MiDOG before you book your next appointment!
References
[1] Vapniarsky, N., Simpson, D., Arzi, B., Taechangam, N., Walker, N., & Garrity, C. et al. (2020). Histological, Immunological, and Genetic Analysis of Feline Chronic Gingivostomatitis. Frontiers In Veterinary Science, 7. doi: 10.3389/fvets.2020.00310
[2] Lee, D. B., Verstraete, F., & Arzi, B. (2020). An Update on Feline Chronic Gingivostomatitis. The Veterinary clinics of North America. Small animal practice, 50(5), 973–982. https://doi.org/10.1016/j.cvsm.2020.04.002
[3] Krumbeck, J., Reiter, A., Pohl, J., Tang, S., Kim, Y., & Linde, A. et al. (2021). Characterization of Oral Microbiota in Cats: Novel Insights on the Potential Role of Fungi in Feline Chronic Gingivostomatitis. Pathogens, 10(7), 904. doi: 10.3390/pathogens10070904
[4] Peralta, S., & Carney, P. (2019). Feline chronic gingivostomatitis is more prevalent in shared households and its risk correlates with the number of cohabiting cats. Journal Of Feline Medicine And Surgery, 21(12), 1165-1171. doi: 10.1177/1098612×18823584
[5] Dolieslager, S., Riggio, M., Lennon, A., Lappin, D., Johnston, N., Taylor, D., & Bennett, D. (2011). Identification of bacteria associated with feline chronic gingivostomatitis using culture-dependent and culture-independent methods. Veterinary Microbiology, 148(1), 93-98. doi: 10.1016/j.vetmic.2010.08.002
[6] Reiter, A. (2018). Dental Disorders of Cats – Cat Owners – Merck Veterinary Manual. Retrieved 21 July 2021, from https://www.merckvetmanual.com/cat-owners/digestive-disorders-of-cats/dental-disorders-of-cats
[7] Druet, I., & Hennet, P. (2017). Relationship between Feline calicivirus Load, Oral Lesions, and Outcome in Feline Chronic Gingivostomatitis (Caudal Stomatitis): Retrospective Study in 104 Cats. Frontiers In Veterinary Science, 4. doi: 10.3389/fvets.2017.00209
[8] Tsang, W., Linde, A., Krumbeck, J., Wu, G., Kim, Y., Lushington, G. and Melgarejo, T., 2021. Occurrence of Antimicrobial Resistance Genes in the Oral Cavity of Cats with Chronic Gingivostomatitis. Animals, 11(12), p.3589.
[9] Sturgeon, A., Pinder, S., Costa, M., & Weese, J. (2014). Characterization of the oral microbiota of healthy cats using next-generation sequencing. The Veterinary Journal, 201(2), 223-229. doi: 10.1016/j.tvjl.2014.01.024
Categories: Antibiotic Resistance, Cats, Next-Gen DNA Sequencing Technology, Oral/Mouth Infections

