
Case Study: Canine Urinary Tract Infection
Discover unsuspected culprits with the MiDOG® All-in-One Microbial Test.
The results here are from a female dog suffering from a long-term urinary problem that had failed to respond to her treatments. Urine samples were sent to both the MiDOG® testing facility and for culture testing. Here are the results:
Culture Testing By an Independent Laboratory
1 isolate: Enterococcus faecalis (10,000 – 50,000 CFU per ml)
Results: Only E. faecalis was cultured successfully.
Comments: Key pathogens like Mycoplasma sp. cannot be cultured easily and were thus missed upon analysis in the culture test.
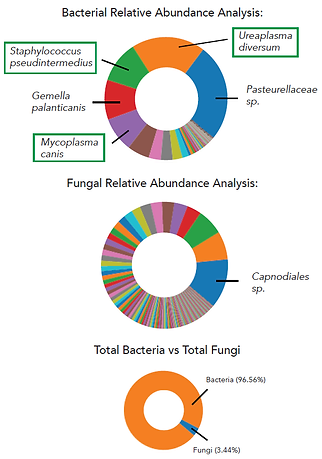
Results: The MiDOG® All-in-One Microbial test clearly revealed that the infection was not caused by fungi, but by bacteria: Several pathogens were identified, including Mycoplasma canis, Staphylococcus pseudintermedius, and Ureaplasma diversum. These three organisms were the most dominant infectious agents in the sample.
Comments: The test identified the key pathogens causing the infection and indicated their absolute abundance as well as the corresponding antibiotic resistance to guide the treatment. Enterococcus, the only isolate from the culture test, was detected at 0.05% relative abundance, but the correct genetic identity was E. feacium, not E. faecalis. As a result of the MiDOG® All-in-One Microbial Test the treatment regimen was changed for a full recovery.
Case Report: A novel DNA sequencing tool for the diagnosis and treatment of urinary tract infections
History:
Daisy, a 5-year-old mixed breed, spayed female dog, was suffering from intermittent urinary tract infections (UTIs) since July 2016. She was presented with frequent urination, straining while urinating, and hematuria with several days duration. Otherwise, she had been acting normally and had no prior history of any serious health concerns. Her physical examination was generally normal with no abnormalities. She weighed 22 pounds, her temperature was between 101 degrees, her heart rate at 110 beats per minute, and her respiration rate between 35 breaths per minute.

Diagnostic Tests and Initial Treatment
All of Daisy’s urine samples reported here have been collected via cystocentesis and all urine analyses were performed by the same reference laboratory. Daisy’s laboratory results for the past three years are summarized here. The urinalysis in July 2016 showed a normal urine pH (6.5), turbid appearance, high amounts of leukocytes and erythrocytes (4-10 and 11-20 high Power Field (HPF), respectively), struvite crystals (2-3 HPF), as well as bacterial cocci (51-100 HPF). The average age of canine patients with struvite bladder stones is 2.9 years, and Daisy was 3 years old at the time [1]. Daisy was treated with Simplicef®2 (Zoetis) at 5 mg/kg q24 hours for three weeks. After the antibiotic treatment she showed no straining while urinating or any other symptoms. She was prescribed a urinary diet (Hill’s Prescription Diet c/d) that she continues to this day, and culture testing showed no bacterial growth when samples were sent to the local reference lab (Late July 2016). Daisy’s urinary tract infection was considered cleared, until she was presented back with severe symptoms less than three months later (October 2016). She had repeatedly urinated in the house, was straining while urinating, and had blood in her urine. A urine sample was collected and analyzed as previously described. This second urinalysis showed a high urinary pH (7.5), high levels of protein, leukocytes (4-10 HPF), struvite crystals (4-10 HPF), and high amounts of bacteria (cocci >100 HPF). Based on her behavior (which was noted above) and laboratory results, her UTI appeared to be more severe than before. Her previous treatment with Simplicef®2 (Zoetis) was repeated and a follow-up hospital visit scheduled for four weeks later to monitor her progress. Daisy’s previously described behavior was not improving, and a urinalysis was ordered again. Her test results in November 2016 were further elevated compared to the previous ones (further increase in urinary pH, higher amount of protein and white blood cells in urine, number of cocci still high). It should be noted that Daisy’s owner had limited financial resources and was not able to afford the additional cost of culture testing, radiographs, or ultrasounds to test for stones or a tumor. Therefore, Daisy’s diagnosis was solely based on urinalysis, physical examination, and behavior.
Over the course of two and a half years, Daisy had nine episodes of urinary tract infections (July 2016 – February 2019). She was treated with steroid (dexamethasone 2mg/ml at 0.2mg/kg) and antibiotics (Simplicef®2 (Zoetis) as noted above), with repeated follow-ups for physical exams and urinalysis in between the episodes. When her owner was able to afford culture testing, the results repeatedly came back ‘no growth’ after the antibiotic treatments.
Treatment Based on New Technology
In February 2019 Daisy’s UTI returned. Her urine was sent for urine culture testing, including antibiotic resistance testing, to the same reference lab as before and, in parallel, was also analyzed using the DNA sequencing technology provided by MiDOG that identifies and quantifies all microorganisms independent of culturing. The MiDOG All-in-One Test kit provides a DNA preservative within the sample collection kit that preserves all microbial DNA at the time of collection. It is the same technology that is currently being used by researchers aboard the International Space Station and by the US Army. This preservative does not require the sample be kept cold prior to it being processed at the MiDOG facility in Irvine, CA. All bacterial and fungal DNA is extracted from the sample and analyzed using Next-Generation DNA sequencing. Unlike PCR testing, this technique is not limited to any number of species or targets and has been widely used to analyze microbial profiles (communities) in environmental and clinical settings [3].

The reference laboratory culture testing revealed moderate growth of Staphylococcus pseudintermedius, with sensitivity to all tested antibiotics. The MiDOG® report detected several bacteria and fungi in Daisy’s urine sample, including two pathogens: Enterococcus faecalis (2 x 107 cells/ml) and Staphylococcus pseudintermedius (7 x 107 cells/ml). S. pseudintermedius was sensitive to all tested antibiotics confirming the results of the culture testing. The E. faecalis species in Daisy’s urine however carried an intrinsic resistance to Simplicef®2 (Zoetis), a cefpodoxime antibiotic. Based on these results it is more than likely, that the previous Simplicef®2 treatment cleared the S. pseudintermedius infection but allowed E. faecalis to continue causing the urinary tract infection over the past years. Enterococcus faecalis is a Gram-positive bacterium that is facultative anaerobe. Even though it very commonly causes canine UTIs [4], it can easily be missed by culture testing. By analyzing the microbial profiles in urine using DNA sequencing instead of culturing, it was easily detected.
Based on the findings of the MiDOG® report, Daisy’s treatment was changed to 2.75 mg/kg q24 hours of marbofloxacin for three weeks. At her follow-up visit in May 2019 Daisy was alert, responsive, and showed no sign of discomfort while urinating. A secondary MiDOG® analysis confirmed that the microbial load in her urine (collected via cystocentesis) was below the detection limit and no pathogens were identified.
Daisy continues to be regularly monitored using the MiDOG® urine analysis test every month. Up to the publication date of this report (October 2019), Daisy has had no more episodes of urinary tract infection.
Bio:
Dr. Kavanagh graduated from Veterinary School at Western University College of Veterinary Medicine in Pomona California in 2007. He is the owner of Saddleback Animal Hospital in Tustin California where he works together with his wife Melinda. He is the proud father of three teenage girls.
References:
1. Wendy Brooks, DVM, DABVP, 10/12/2017. Bladder Stones (Struvite) in Dogs. VeterinaryPartner. https://veterinarypartner.vin.com/doc/?id=4951352&pid=19239
2. https://www.zoetisus.com/contact/pages/product_information/msds_pi/pi/simplicef.pdf
3. Weinstock et al. 2016; A Report from the American Academy of Microbiology. Applications of clinical microbial next-generation sequencing.
4. Kate S. KuKanich and Brian V. Lubbers (2015) Review of Enterococci Isolated from Canine and Feline Urine Specimens from 2006 to 2011. Journal of the American Animal Hospital Association: May/June 2015, Vol. 51, No. 3, pp. 148-154.
Case Report: Sea lion pup Hai-Xi, a tale of resilience

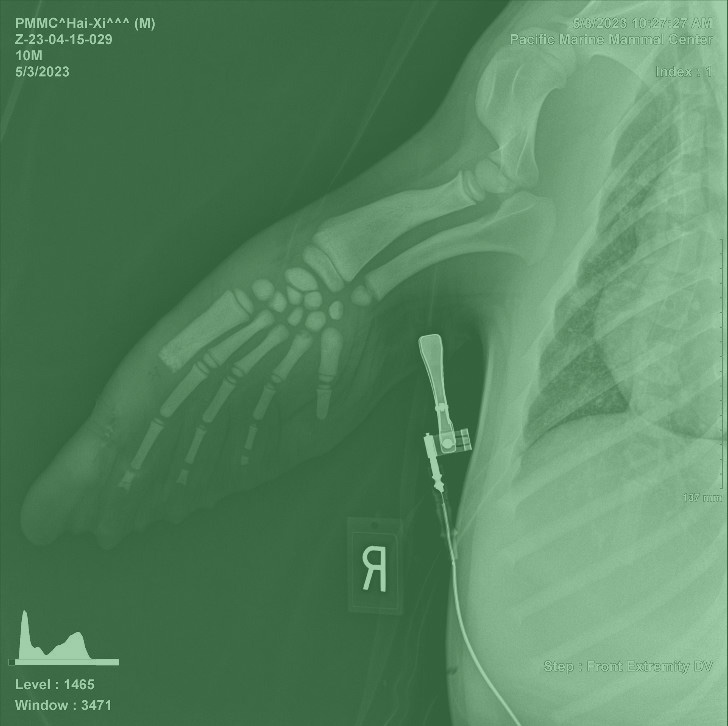
In spring 2023 Hai-Xi, a 9-month-old California sea lion pup discovered stranded along the shores of Seal Beach, CA (Picture 1). Dealing with dehydration and malnutrition, he weighed a mere 39 lbs. upon rescue. Despite a noticeable limp during his rescue, Hai-Xi remained stoic as he was transported in a rescue kennel to the Pacific Marine Mammal Center (PMMC) in Laguna Beach, CA. Upon arrival, swelling in his right front flipper raised concerns of a potential wound or abscess.
Under the care of the PMMC veterinary team, Hai-Xi underwent a thorough examination, including radiographs and comprehensive bloodwork. Treatment began promptly with rehydration, nutritional support, and tailored medication regimens. Unfortunately, the wound on his right front flipper deteriorated, leading to bone exposure at the tip of his digit (Picture 2). As part of the clinical evaluation, a sample from his wound was sent to MiDOG for the All-in-One Test and revealed a severe infection with Streptococcus phocae, an opportunistic pathogen found in marine mammals (1-5). This bacterium can be acting as a commensal in some individuals, but in Hai-Xi’s case, over 40 million cells were detected representing almost 100% of his microbiome (Picture 3). Further, this Gram-positive aerobic fish pathogen (6) carried resistances to multiple antibiotics, specifically Gentamicin, Neomycin, Amikacin, Ceftazidime, and Colistin.
After careful evaluation and further imaging, it was decided that amputation of two bones in his thumb was necessary due to severe infection and dislocation. With courage, Hai-Xi underwent successful surgery under general anesthesia, followed by a meticulous closure of the wound (Pictures 4 and 5). A re-check with MiDOG revealed that no Streptococcus phocae cells were present on his skin anymore and the infection was cleared.
Release Day
Play VideoAs days turned into weeks, Hai-Xi’s flipper gradually healed, restoring full functionality. With his weight reaching a healthy level, and after three months of intensive rehabilitation, the time for release had finally arrived. With a clean bill of health, Hai-Xi joyously returned to the embrace of the ocean at Aliso Creek Beach, reuniting with his companions Garchomp and Pinata amidst the vast blue expanse. You can see Hai-Xi’s release video here: Release Day: Pinata, Hai-Xi and Garchomp (youtube.com)
The marine mammals on the coastlines of California face a variety of challenges that necessitate assistance and intervention, including pollution, habitat degradation, climate change, overfishing, and human interaction.
The Pacific Marine Mammal Center is a non-profit organization that rescues marine mammals in need, with many of them suffering from injuries and infections. Please consider donating to the Pacific Marine Mammal Center to support all the amazing work they do for the California sea life: Rescue | Rehabilitate | Release

References:
- Taurisano, Nicole D., et al. “Streptococcus phocae in marine mammals of northeastern Pacific and Arctic Canada: a retrospective analysis of 85 postmortem investigations.” Journal of wildlife diseases 54.1 (2018): 101-111.
- Carpenter, James W., and Chris Marion. Exotic Animal Formulary-E-Book. Elsevier Health Sciences, 2017.
- Wallach, Joel D., and William J. Boever. Diseases of exotic animals. Medical and surgical management. WB Saunders Co., 1983.
- Ballard, Bonnie, and Ryan Cheek, eds. Exotic animal medicine for the veterinary technician. John Wiley & Sons, 2016.
- Abstract: Denise M. Imai; Spencer S. Jang; Melissa A. Miller; Janet E. Foley; Patricia A. Conrad. “Characterization of Streptococcus phocae and Streptococcus dysgalactiae Sp. Isolated from Southern Sea Otters (Enhydra lutris nereis)” IAAAM Archive, https://www.vin.com/apputil/project/defaultadv1.aspx?pid=11257&catid=&id=3865148&meta=&authorid=
- Gibello, A., Mata, A. I., Blanco, M. M., Casamayor, A., Domínguez, L., & Fernández-Garayzabal, J. F. (2005). First identification of Streptococcus phocae isolated from Atlantic salmon (Salmo salar).Journal of clinical microbiology,43(1), 526-527.
Avian Gastrointestinal Infection

A 29-year-old, female Congo African grey parrot (Psittacus erithacus) was presented for a 2-month history of soft malodorous stool. Next-generation DNA sequencing of the faecal matter identified Anaerosporobacter sp. in the stool sample. The bird was treated with a 14-day course of metronidazole, after which next-generation DNA sequencing no longer detected Anaerosporobacter sp. The bird’s clinical signs resolved concurrently. Clinical signs of malodorous stool recurred, and repeated next-generation DNA sequencing showed arecurrence of Anaerosporobacter sp. Duodenal dysmotility/hypermotility syndrome was noted on fluoroscopy. The bird also tested positive for bornavirus and anti-ganglioside antibodies. We suspect that dysmotility and hypermotility of the duodenum were associated with active bornavirus infection, which caused immunosuppression and predisposed the bird to intestinal colonisation by a novel organism. This report demonstrates the presumptive diagnosis and treatment challenges of Anaerosporobacter sp. from the faeces of a psittaciform species using next-generation DNA sequencing as opposed to traditional culture techniques.
Citation: Vecere, G., Malka, S., Dennison, S., & Krumbeck, J. (2024). Identification of Anaerosporobacter sp. using next‐generation DNA sequencing as a presumptive cause of chronic cloacitis in a Congo African grey parrot (Psittacus erithacus). Veterinary Record Case Reports, 12(1), e749.

Validated by Veterinarians
